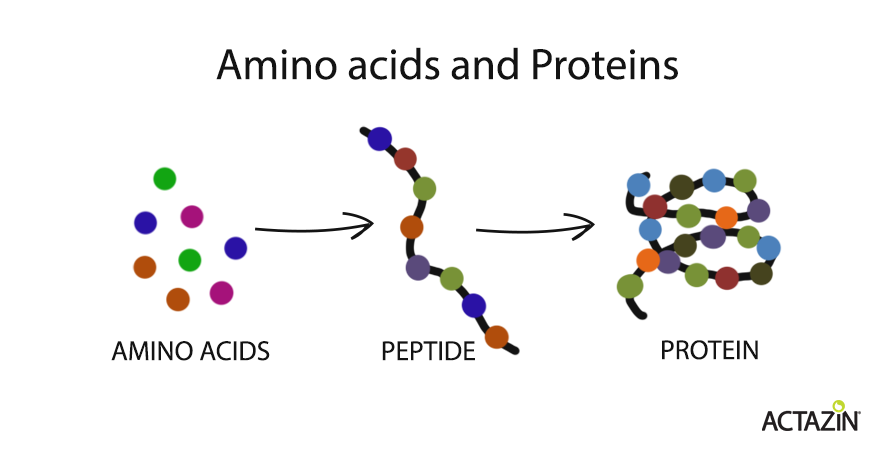
What is protein and how is it digested?
Proteins are essential components of the human body – our muscle, skin, hair, hormones, and enzymes, for example, are made from protein. Proteins are polymers (polypeptides) made up of sequences/chains of small organic compounds called amino acids. A chain of less than 30 amino acids is referred to as a peptide. There are 20 amino acids that form the building blocks for proteins, but the body can only make 11 of these – the other nine, known as essential amino acids, must come from the diet.
Protein is one of the three macronutrients in our diet, the other two being carbohydrate and fat. Dietary sources of protein include meat, dairy, fish, eggs, legumes, nuts and grains.
The digestion of dietary proteins in humans begins in the stomach where the initial breakdown is done via the action of pepsin (a protease) and hydrochloric acid (stomach acid). The resulting peptides and protein fragments make their way to the small intestine where further enzymes (e.g. trypsin, chymotrypsin) continue to digest the peptides and fragments into amino acids and small peptides, which can then be absorbed into the bloodstream.
Once absorbed the amino acids and small peptides are utilised as building blocks by cells in the body to create new proteins, such as enzymes, antibodies, structural proteins (e.g. keratin, collagen and elastin), and messenger proteins (e.g. hormones), or they can be broken down to produce energy.
Poor protein digestion
Some dietary proteins are more readily digested than others and the presence of a significant amount of poorly digested dietary protein in the stomach can reduce the rate of emptying of the stomach leading to that overfull feeling/bloating. This is particularly an issue for people with compromised digestion, such as elderly people, who tend to produce less pepsin and/or stomach acid, or people on proton pump inhibitor drugs, which reduce the production of acid in the stomach.
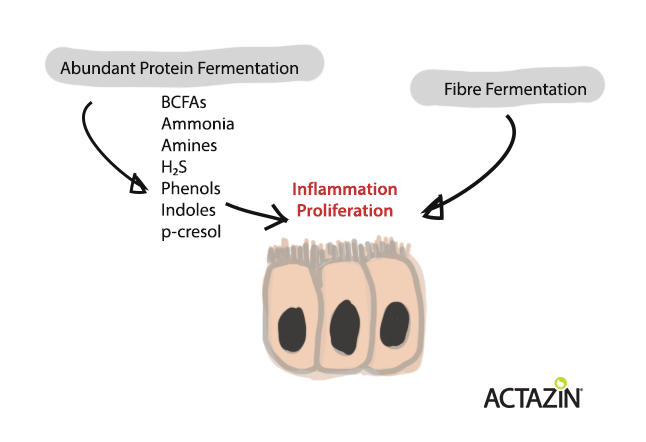
Undigested protein and peptides that reach the colon are fermented by the gut microbiota. Fermentation of protein produces several byproducts including ammonia, hydrogen sulphide, short chain fatty acids (SCFA), branched chain fatty acids (BCFA), amines, phenols and indoles. Too much protein fermentation (which can occur with high protein diets or poor digestion of protein) can result in an overabundance of the fermentation byproducts which may cause inflammation (potentially leading to a “leaky gut”) and excessive proliferation of colonocytes (potentially leading to colorectal cancer) [1]. Increased fibre fermentation in the colon with increased SCFA (especially butyrate) production however can be protective against colorectal cancer development.
Protein intolerance/sensitivity
Some people are also intolerant or sensitive to certain proteins. The most well-known is gluten intolerance/sensitivity, but casein may also pose an issue for some.
Casein intolerance
Casein is the major milk protein. Research suggests the A1 type of beta may cause intolerance reactions [2,3]. In contrast, the A2 type and whey (the minor milk protein) have not been associated with reactions and appear to be well-tolerated [4,5].
When casein is digested, an opiate-like substance, beta-casomorphin-7, is released. It is thought that this compound is responsible for the intolerance reactions observed with casein by stimulating histamine release [3]. Histamine is involved in the inflammatory response to allergens.
Gluten Intolerance
Many people experience digestive and other health problems when they eat gluten/wheat. Gluten is a group of proteins found in wheat, barley and rye. Wheat is used as an ingredient in a large number of foods, including breads, pastas, cereals, biscuits, crackers, cakes and pastries, but it also shows up in a lot of convenience foods, like soups, sauces, spices, drinks, processed meats and ready-made meals. Barley is mainly used in beer and foods containing malt, but can also be found in soups (e.g. pearled barley). Rye is found in bread, crackers, cereals, beer and whiskey.
Gluten intolerance is experienced by a significant proportion of the population with prevalence rates of up to 13% [6]. Gluten intolerance can present as:
- Celiac disease (CD) – an autoimmune condition whereby the ingestion of gluten causes damage to the small intestine.
- Non-celiac gluten sensitivity (NCGS) – a condition that produces symptoms similar to those seen in CD, however unlike CD there is no damage to the small intestine, it is not autoimmune, nor does it have a genetic component.
- Wheat allergy
The adverse effects of gluten are understood to be largely due to a reaction to proline-rich peptides that are not normally digested in the gut. The gluten proteins, gliadins and glutenins, are very resistant to gastrointestinal proteases (protein-digesting enzymes), resulting in the presence of the indigestible peptides which can be immunogenic (cause an immune response).
What can we do to enhance protein digestion?
To help your body digest protein effectively you can try:
- Eating a balanced diet and spread out your protein intake during the day.
- Ensuring you chew your food properly before swallowing – chewing is the first part of the digestive process. Not only does it signal to the brain that you are eating, it helps to break the food down into more manageable pieces for your digestive enzymes.
- Eating foods that contain proteases, for example, kiwifruit, pineapple and figs.
Natural fruit proteases, like actinidin from kiwifruit, has been shown to enhance the digestion of a range of proteins, including meat proteins, casein and gluten. For gluten in particular, actinidin is also effective in hydrolysing (breaking down) the resistant gluten peptides [7]. Further research has shown that the presence of actinidin can increase the rate of stomach emptying, reducing that feeling of overfullness after a high protein meal and potentially increasing the rate of amino acid uptake and utilisation. Actazin® is a green kiwifruit powder rich in actinidin. For more information on actinidin, check out our blog: Actinidin: a kiwifruit unique, protein-digesting enzyme.
References:
- Diether, N., & Willing, B. (2019). Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms, 7: 19.
- Haq, ,., Kapila, R., .Sharma, R., Saliganti, V., & Kapila, S. (2014). Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th 2-mediated inflammatory response in mouse gut. European journal of nutrition, 53(4), 1039-1049.
- Brooke-Taylor, S., Dwyer, K., Woodford, K., & Kost, N. (2017). Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 b-Casein. Advances in Nutrition, 8(5): 739-478.
- Jianqin, S., Leiming, X., Lu, X., Yelland, G., & Clarke, A. (2015). Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutrition Journal, 15: 35.
- He, M., Sun, J., Jiang, A., & Yan, Y. (2017). Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutrition Journal, 16: 72.
- Molina-Infante, J., Santolaria, S., Sanders, D., & Fernandez-Banares, F. (2015). Systematic Review: non-celiac gluten sensitivity. Alimentary Pharamcology and Therapeutics, 41: 807-820.
- Jayawardana, I., Boland, M., Higgs, K., Zou, M., Loo, T., McNabb, W., & Montoya, C. (2021). The kiwifruit enzyme actinidin enhances the hydrolysis of gluten proteins during simulated gastrointestinal digestion. Food Chemistry 341, 128239.